Climate change encourages the energy sector to replace conventional energy sources, which produce a high amount of CO2 emissions, with renewable alternatives. In addition, industries are aiming to reduce their energy losses from waste heat. This increases the attractiveness of storage systems and boosts the research on this topic. An additional challenge is storing heat that is produced in the summer until the winter, when the energy demand is higher. As a result, storage systems that can deposit energy heat loss-free over a long period of time, are particularly in demand. Storing energy in chemical reactions, also known as thermochemical energy storage (TCES), meets this requirement. Moreover it convinces through high energy densities in comparison to other thermal energy storage systems.
The principle of a thermochemical energy storage
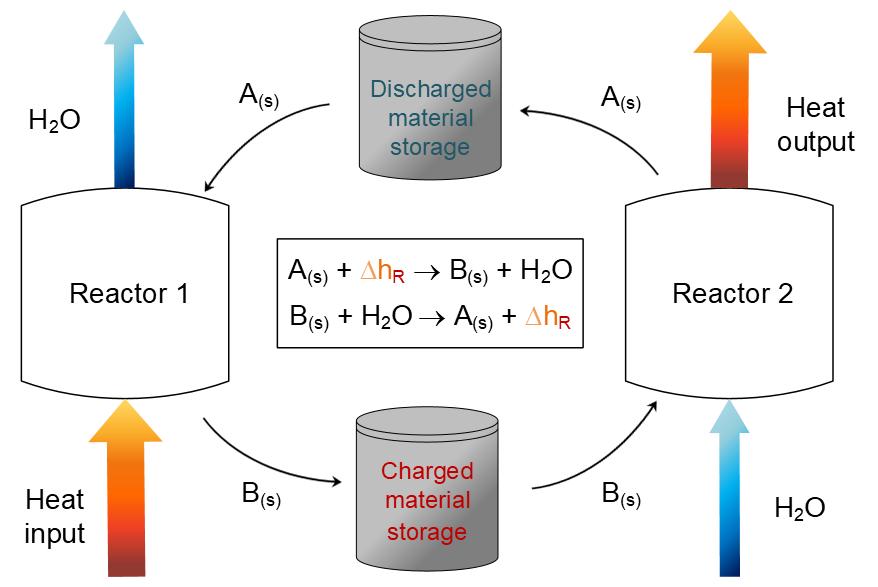
The general TCES principle is as follows (see figure): when charging the storage unit, heat is added to an endothermic reaction resulting in products, that are then stored separately. Once the energy is needed, the products can be combined again in order to release the reaction enthalpy.
The process can be operated in a continuous mode when using an appropriate reactor concept. The charging and discharging cycles can be conducted in two separate reactors, using storage tanks in between. In the first reactor, the charging reactor, heat is added. The solid component A reacts to solid component B and the gaseous component, in this case water vapour, is released. After a storage period, component B enters the second reactor, to which water is added. This allows a release of the stored energy. The thermochemical cycle is then completed and can be started again, by transferring component A back into reactor 1.
Requirements to the TCES materials
Materials that are used for TCES must meet several criteria: they are supposed to be non-expensive and non-toxic, and their reactions should be reversible, ensure cycle stability and take place in a certain temperature range, in this case up to 200°C. This enables the storage system to store low to middle temperature heat, such as solar energy as well as industrial waste heat. Possible areas of application within this temperature range are in district heating, the food industry or paper production. Examples for reactants that fulfil those requirements are various salt hydrates as well as boric acid (H3BO3).
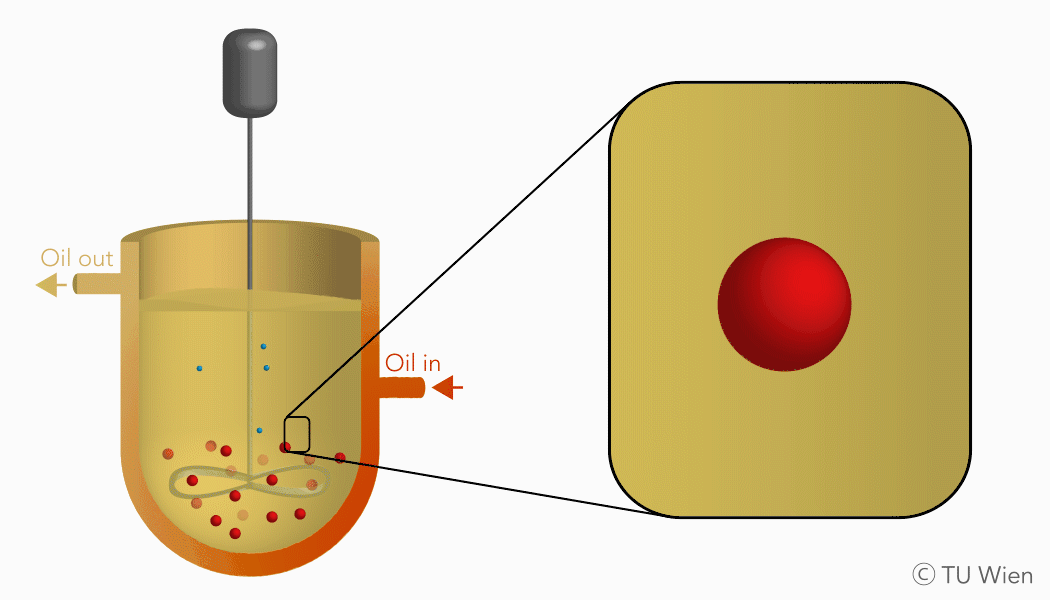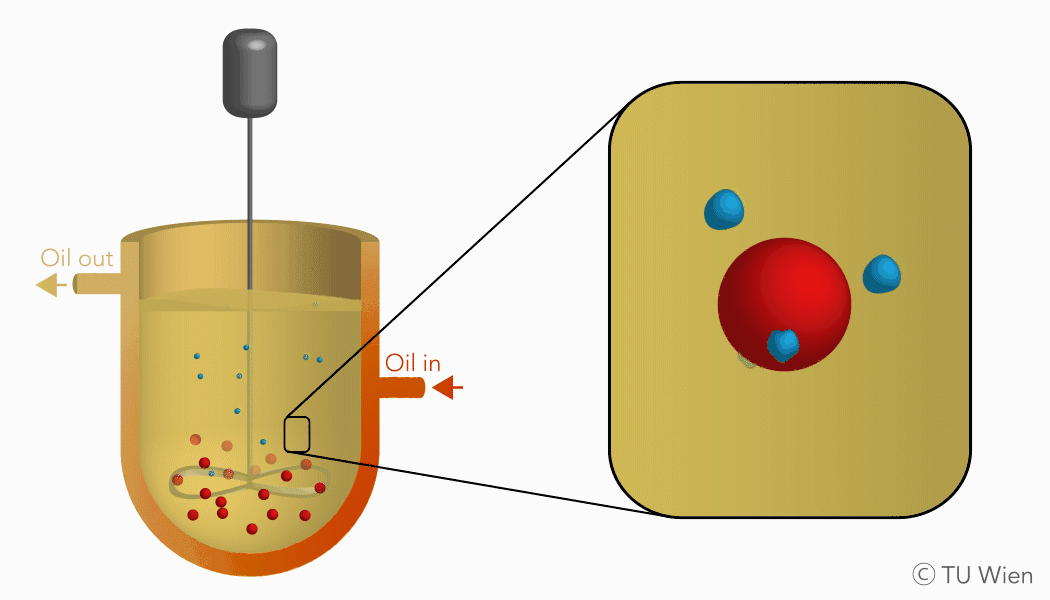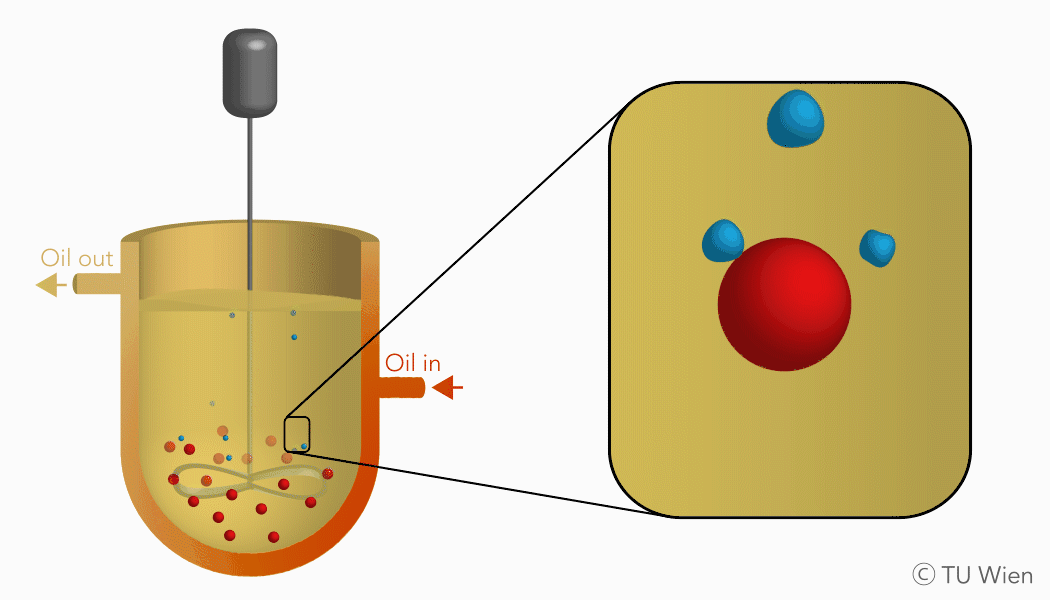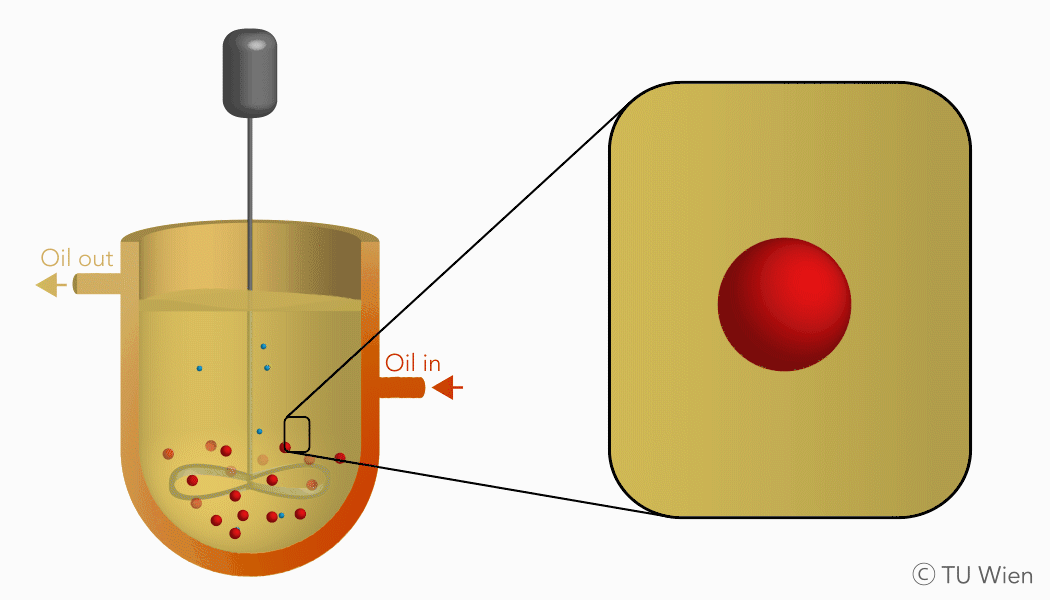
The mentioned solid reactants are used in a three-phase suspension reactor which harbours several advantages, as the liquid suspension supports heat and mass flow. Currently the experimental setup consists of a stirred reactor with thermal oil as the suspension medium. Inside, the solid reactants are suspended, heated, and thus converted through the mentioned gas-solid reaction. The gaseous product, water vapour, is released, condensed, and collected elsewhere. The solid product remains in the suspension and is stored until its further use.
Charging reaction of the TCES system in a three-phase suspension reactor (click to enlarge)